Version: 1.0.0 | Published: 10 Apr 2024 | Updated: 689 days ago
Documentation
Associated Media:
Description:
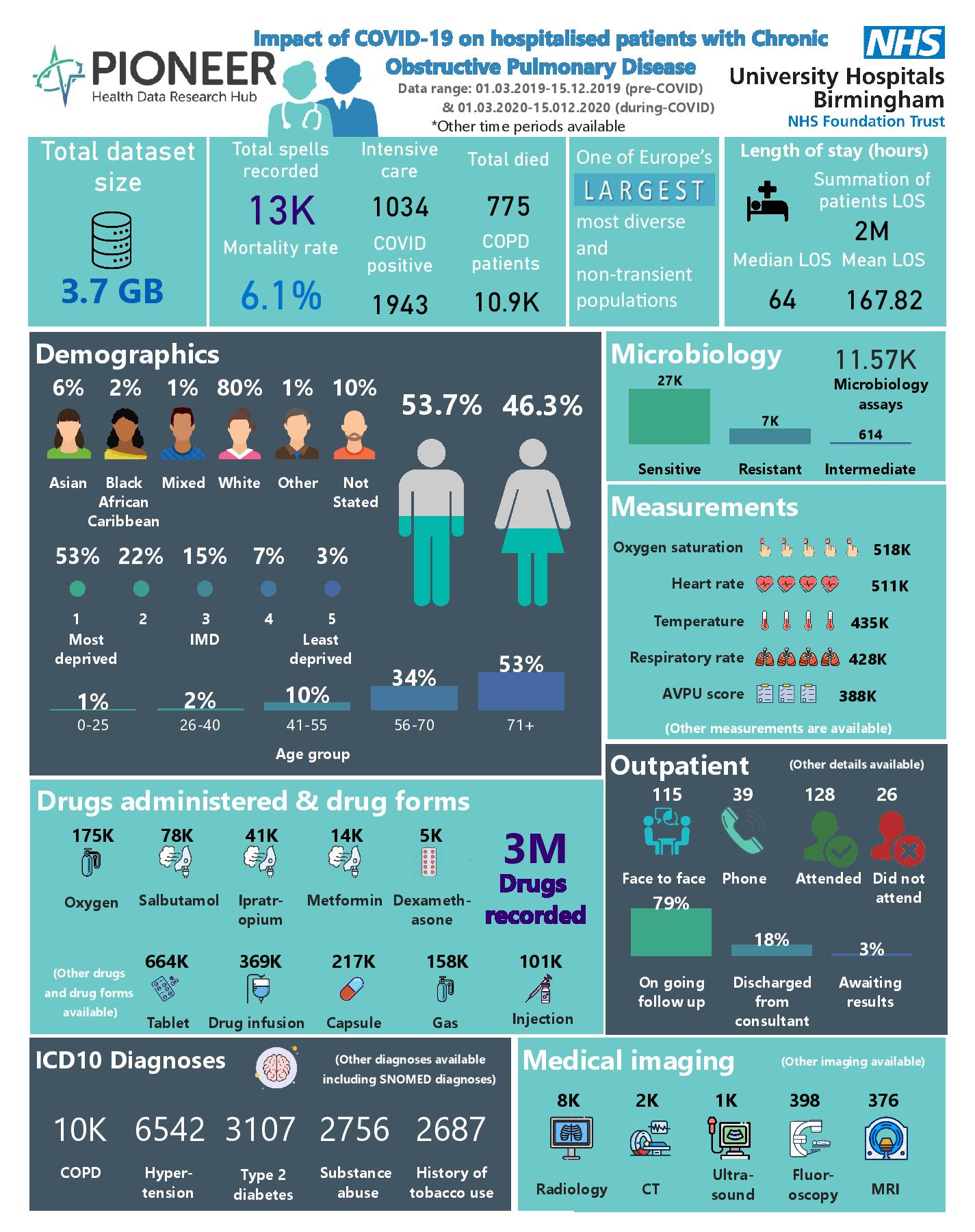
Chronic obstructive pulmonary disease (COPD) is a debilitating lung condition characterised by progressive lung function limitation. COPD is an umbrella term and encompasses a spectrum of pathophysiologies including chronic bronchitis, small airways disease and emphysema. COPD caused an estimated 3 million deaths worldwide I each year, and is estimated to be the third leading cause of death worldwide. The British Lung Foundation (BLF) estimates that the disease costs the NHS around £1.9 billion per year. COPD is therefore a significant public health challenge. This dataset explores the impact of hospitalisation and service use in patients with COPD during the COVID pandemic.
PIONEER geography The West Midlands (WM) has a population of 5.9 million & includes a diverse ethnic & socio-economic mix.
EHR. UHB is one of the largest NHS Trusts in England, providing direct acute services & specialist care across four hospital sites, with 2.2 million patient episodes per year, 2750 beds & an expanded 250 ITU bed capacity during COVID. UHB runs a fully electronic healthcare record (EHR) (PICS; Birmingham Systems), a shared primary & secondary care record (Your Care Connected) & a patient portal “My Health”.
Scope: All hospitalised patients admitted to UHB during the COVID-19 pandemic and elective service users, curated to focus on COPD. Longitudinal & individually linked, so that the preceding & subsequent health journey can be mapped & healthcare utilisation prior to & after admission understood. The dataset includes highly granular patient demographics & co-morbidities taken from ICD-10 & SNOMED-CT codes. Serial, structured data pertaining to acute care process (timings, staff grades, specialty review, wards), presenting complaint, acuity, all physiology readings (pulse, blood pressure, respiratory rate, oxygen saturations), all blood results, imaging reports, all prescribed & administered treatments (fluids, blood products, procedures), all outcomes.
Available supplementary data: Matched controls; ambulance, OMOP data, synthetic data.
Available supplementary support: Analytics, Model build, validation & refinement; A.I.; Data partner support for ETL (extract, transform & load) process, Clinical expertise, Patient & end-user access, Purchaser access, Regulatory requirements, Data-driven trials, “fast screen” services.
Is Part Of:
NOT APPLICABLE
Coverage
Spatial:
United Kingdom, England, West Midlands
Typical Age Range:
15-110
Follow Up:
1 - 10 YEARS
Physical Sample Availability:
NOT AVAILABLE
Pathway:
Data is representative of the multi-ethnicity population within the West
Midlands (42% non white). Data includes all patients admitted during this
timeframe, with National data Opt Outs applied, and therefore is representative
of admissions to secondary care. Data focuses on in-patient stay in hospital
during the acute episode but can be supplemented on request to include previous
and subsequent hospital contacts (including outpatient appointments) and
ambulance, 111, 999 data.
Provenance
Origin
Purposes:
CARE
Sources:
EPR
Collection Situations:
- ACCIDENT AND EMERGENCY
- IN-PATIENTS
- OUTPATIENTS
Temporal
Accrual Periodicity:
QUARTERLY
Distribution Release Date:
18 May 2021
Start Date:
01 March 2019
End Date:
15 December 2020
Time Lag:
OTHER
Accessibility
Access
Access Service:
Trusted Research Environments (TRE) are built using Microsoft Azure services and
hosted in the UK to provide research teams a safe, secure and agile environment
which allows users to quickly analyse, interpret and form an enriched view of
primary care information through a range of integrated datasets. Health data
collated from multiple sources is ingested into a secure data lake which will
then allow subsets of data to be made available to research teams on approval of
a data request. Once approved a customer specific TRE is made available with a
standard set of leading analytical tools from Microsoft including Azure
Databricks, Azure Machine Learning, Azure SQL and Azure Synapse (for large-scale
data warehouses). Specific tools can be provided at an additional cost over the
standard platform data access charge and the PIONEER team will work with you to
determine your exact needs. Access to the TRE is managed using the latest
virtual desktop technology to provide a safe and secure end-user experience. By
utilising leading edge design PIONEER are able to create TREs rapidly to enable
us to service any customer requirement.
Access Request Cost:
www.pioneerdatahub.co.uk/data/data-services-costs/
Delivery Lead Time:
1-2 MONTHS
Jurisdictions:
GB-ENG
Data Controller:
University Hospitals Birmingham NHS Foundation Trust
Data Processor:
NOT APPLICABLE
Usage
Data Use Limitations:
GENERAL RESEARCH USE
Data Use Requirements:
PROJECT SPECIFIC RESTRICTIONS
Resource Creators:
- This publication uses data from PIONEER
- an ethically approved database and analytical environment (East Midlands Derby Research Ethics 20/EM/0158)
Is Referenced By:
NOT APPLICABLE
Format and Standards
Vocabulary Encoding Schemes:
- ICD10
- OPCS4
- SNOMED CT
Conforms To:
LOCAL
Languages:
en
Formats:
SQL
Enrichment and Linkage
Derivations:
NOT APPLICABLE
Observations
Statistical Population
Population Description
Population Size
Measured Property
Observation Date
Events
12,691 COPD (both with a positive and negative swab for SARS-CoV-2 infection) and Non COPD patients (with a positive swab for SARS-CoV-2 infection) between 2019-03-01 to 2020-12-15
12691
Count
18 May 2021
